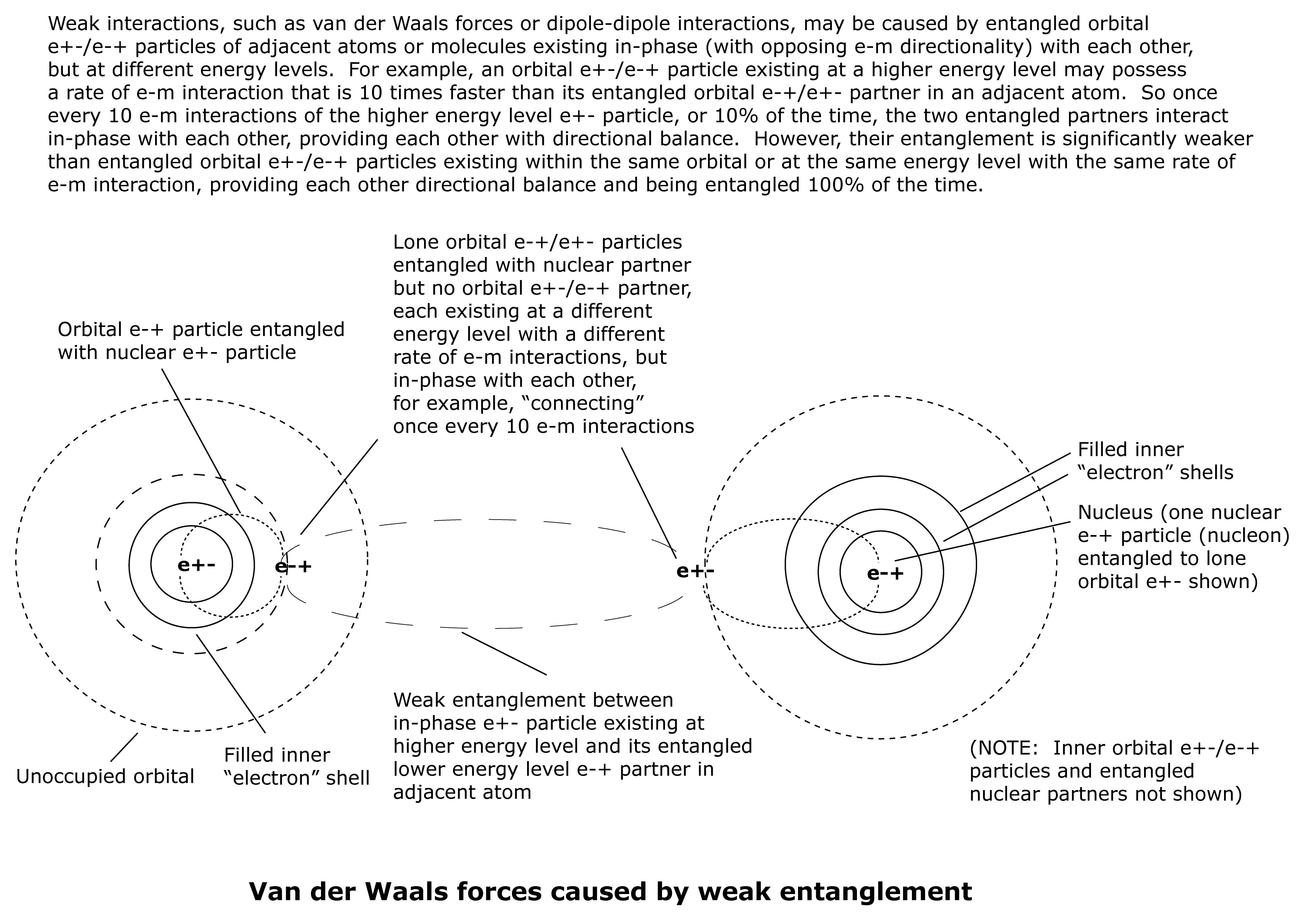
Van der waals force may be due to the entanglement of orbital e+-/e-+ and e-+/e+- particles existing at different energy levels in adjacent atoms. These entangled orbital particles each possess significantly different rates of e-m interaction, but are in-phase (opposing e-m directionality) with each other. This results in a weak entanglement or a weak bond.
Van der waals force may be due to the entanglement of orbital e+-/e-+ and e-+/e+- particles existing at different energy levels in adjacent atoms. These entangled orbital particles each possess significantly different rates of e-m interaction, but are in-phase (opposing e-m directionality) with each other. This results in a weak entanglement or a weak bond.
Each of the orbital e+-/e-+ particles is entangled with a nucelar e-+/e+- particle in their respective nuclei, possessing the same rate of e-m interaction, resulting in a strong entanglement.
Example of orbital e-+/e+- particles involved in creation of Van der Waals force:
If particle A has 10 e-m interactions for every one e-m interaction of particle B, then particle B would experience entanglement with particle A with every e-m interaction, while particle A would only experience entanglement with particle B once out of every ten e-minteractions. This means that particle A would not be entangled with particle B nine out of every ten e-m interactions, giving it greater opportunity to interact with other energy systems (possibly becoming disentangled from particle B) than if the two particles had the same rate of e-m interaction.
Another possibility is that Particle A may also be entangled with up to nine other particles with the same slower rate of e-m interaction as Particle B. In any case, the entangled relationship between Particle A and Particle B is a weak one, resulting in a weak bond between their respective atoms.
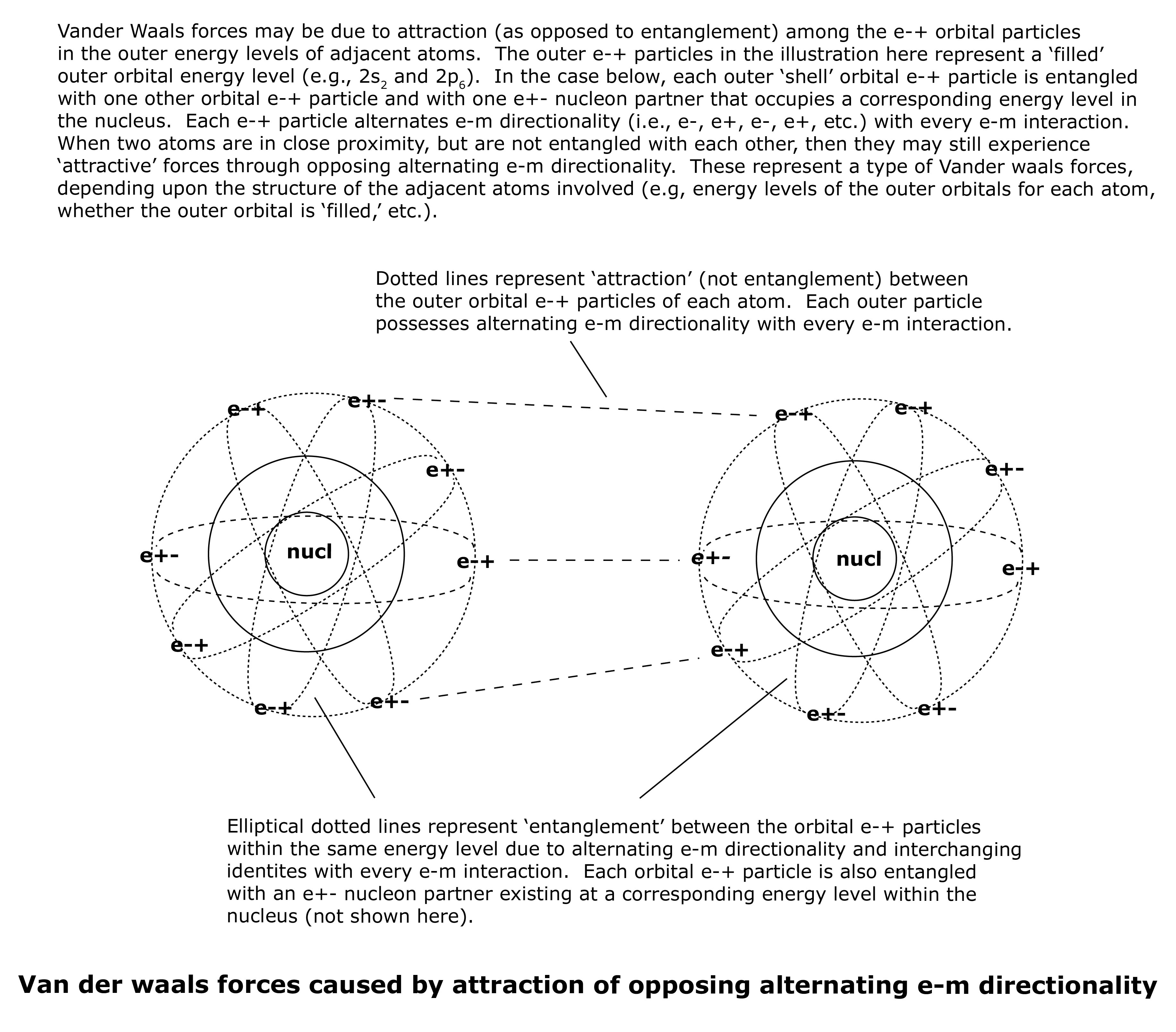
Vander waals forces may also be due to ‘attraction’ (as opposed to ‘entanglement’) among the orbital e-+ particles in two adjacent atoms with opposing e-m directionality. As the adjacent orbital e-+ particles alternate directionality with every e-m interaction, their opposing e-m directionality provides a rather weak, but attractive, force between them. The amount of attraction between the outer orbital e-+ particles of adjacent atoms depends upon a number of factors, including energy levels of the outer orbital ‘shell’ of each adjacent atom, whether the outer orbital energy level (outer ‘shell’) of each adjacent atom is partially ‘filled’ or completely ‘filled,’ etc. See illustration below.