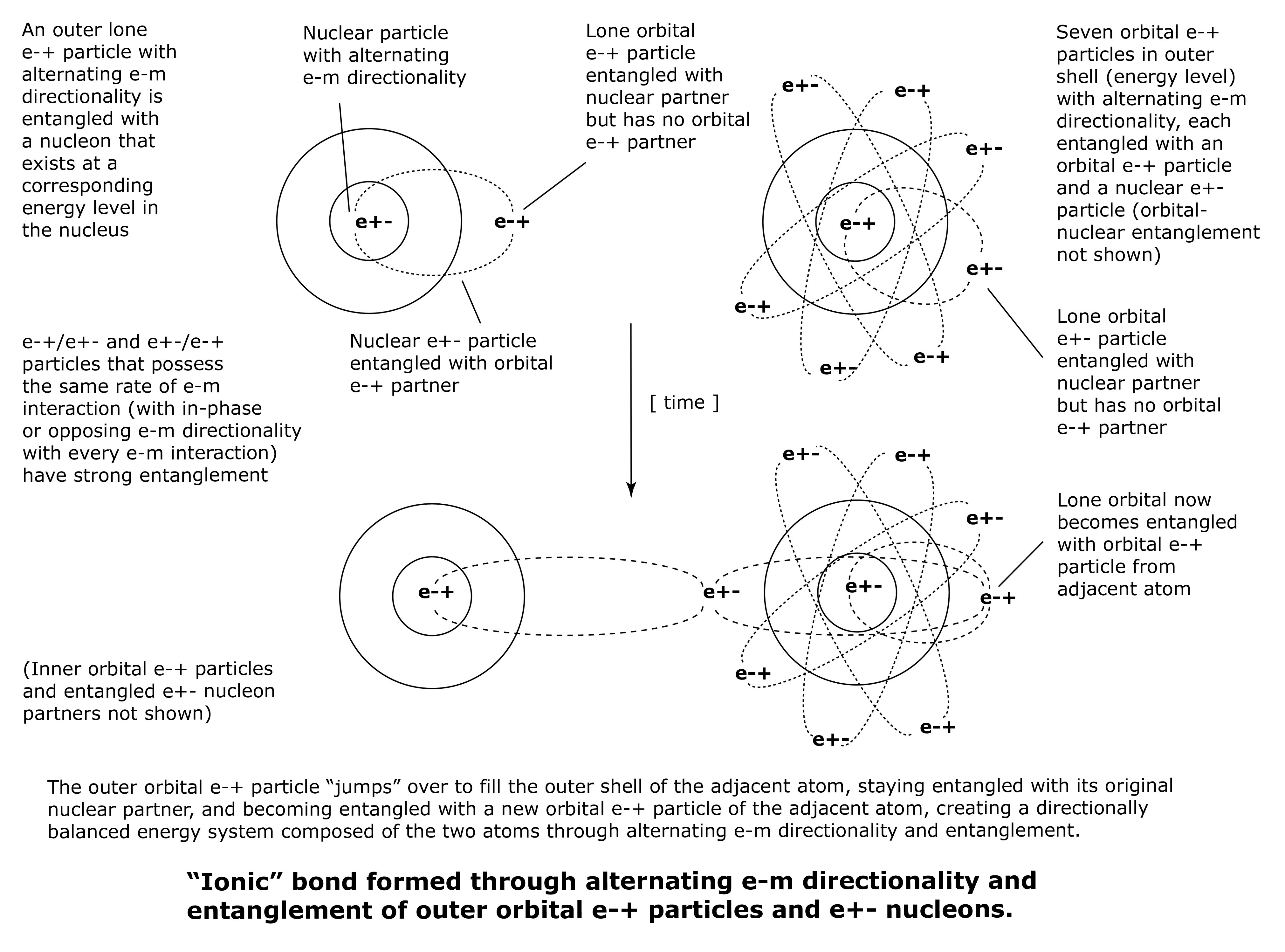
Ionic bonds consist of one unpaired orbital e-+/e+- particle of a metal atom that is entangled with a nuclear e+-/e-+ particle and remains entangled with that partner, but also becomes entangled with an unpaired orbital e+-/e-+ particle in an adjacent non-metal atom.
The lone orbital e-+/e+- particle of the metal atom “jumps” over to fill or complete the outer energy shell of the adjacent non-metal atom, staying entangled with its original nuclear e+-/e-+ partner, and becoming entangled with a new orbital e+-/e-+ particle of the non-metal atom. This creates a directionally balanced energy system composed of a metal atom and a non-metal atom, through the entanglement of their respective outer orbital e-+/e+- particles.
Each of the newly entangled orbital e-+/e+- particles is entangled with a nuclear e+-/e-+ partner in its original atom. These entangled relationships all contribute to the strength of the ionic bond between the two atoms.
See examples of NaCl in crystalline form and Na+Cl- ionic bonding in H2O (currently under review and editing 5/11/21).