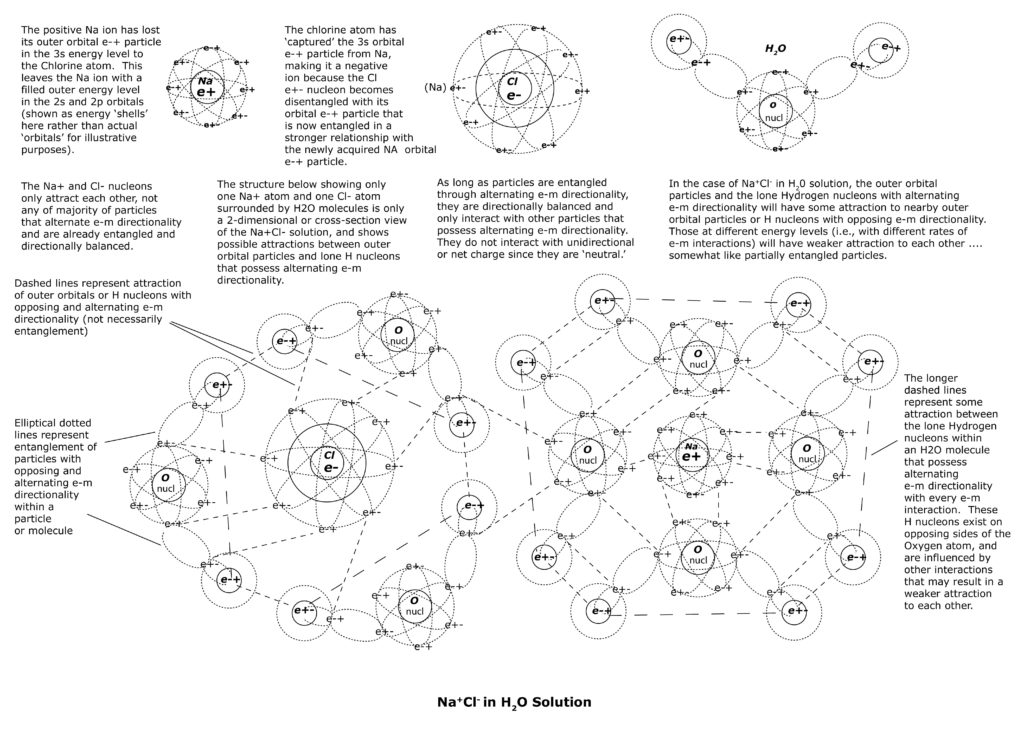
The introduction of NaCl to H2O causes instability due to the addition of numerous possible entangled relationships and attractive forces due to close proximity of orbital e-+ particles with opposing and alternating e-m directionality – that of H2O molecules surrounding Na and Cl atoms (see illustrations below).
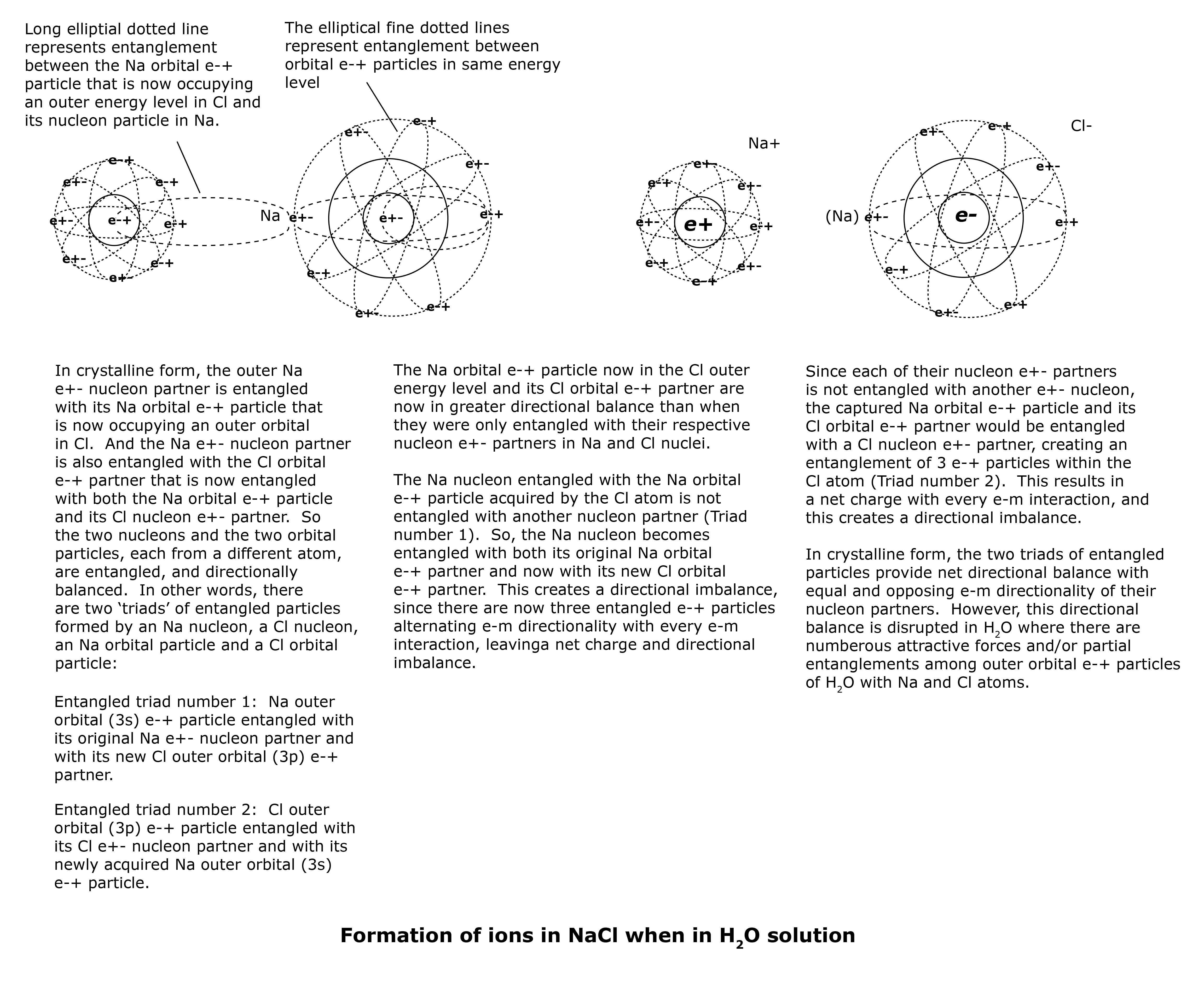
The entanglement of the Na orbital e-+ particle now in the Cl outer energy level and its Cl orbital e-+ partner now possess greater directional balance than when they were when only entangled with their respective nucleon e+- partners in Na and Cl nuclei. This is because the two outer orbital e-+ particles occupy the same orbital with alternating e-m directionality and interchanging identities with every e-m interaction, providing optimal directional balance to the two orbital e-+ particles. As a result, when there is any disturbance causing disentanglement of either of the two ‘triads’ of entangled particles (i.e., 1) Na outer orbital e-+ particle, Na nucleon partner, Cl outer orbital partner; 2) Cl outer orbital e-+ particle, Cl nucleon partner, Na outer orbital partner), the outer orbital particles of Na and Cl will remain entangled, and each will become disentangled from their respective nucleon partners.
The H2O molecules surround the NaCl crystalline structure which gradually ‘dissolves’ into the H2O solution due to attractions among the outer orbital e-+ particles of H2O with those of NaCl. The two sets of entangled triads of NaCl now become less stable. In crystalline form, the two sets of entangled triads of e-+ particles (each with two orbital and one nucleon partners) are stable and directionally balanced due to their net charge being neutralized with every e-m interaction with opposing and alternating e-m directionality of the ‘odd’ particles with every e-m interaction. In other words, when the lone Na nucleon in one entangled triad possesses alternating e-m directionality that opposes that of the Cl nucleon in the other entangled triad, the two nucleons provide each other and the two sets of entangled triads directional balance with every e-m interaction. As a result, the two triads of entangled particles do not possess a net charge with every e-m interaction. NaCl is crystalline form is non-ionic or electrically neutral.
But, in H2O, the multiple attractions and possible partial entanglements among the adjacent outer orbital e-+ particles of H2O molecules with NaCl can easily cause directional imbalance to the two sets of entangled triads within NaCl. It is unlikely that the delicate directional balance among the two sets of entangled triads of NaCl will continue for long in H2O solution as a result.
The attractions of the alternating orbital e-+ particles of H2O molecules disrupt the directional balance of the triads, leaving only the orbital particles of the Na+ ion and Cl- ion entangled and directionally balanced. The unpaired Na nucleon and unpaired Cl nucleon now become disentangled from their respective e-+ orbital partners, and take on net charges without alternating directionality. In other words, the Na nucleon is now positive charged with every e-m interaction and the Cl nucleon is now negatively charged with every e-m interaction. The Na+ ions and Cl- ions are now each surrounded by H2O molecules and interact with each other through attractions or partial entanglements of the outer orbital e-+ particles of each (see illustrations below).