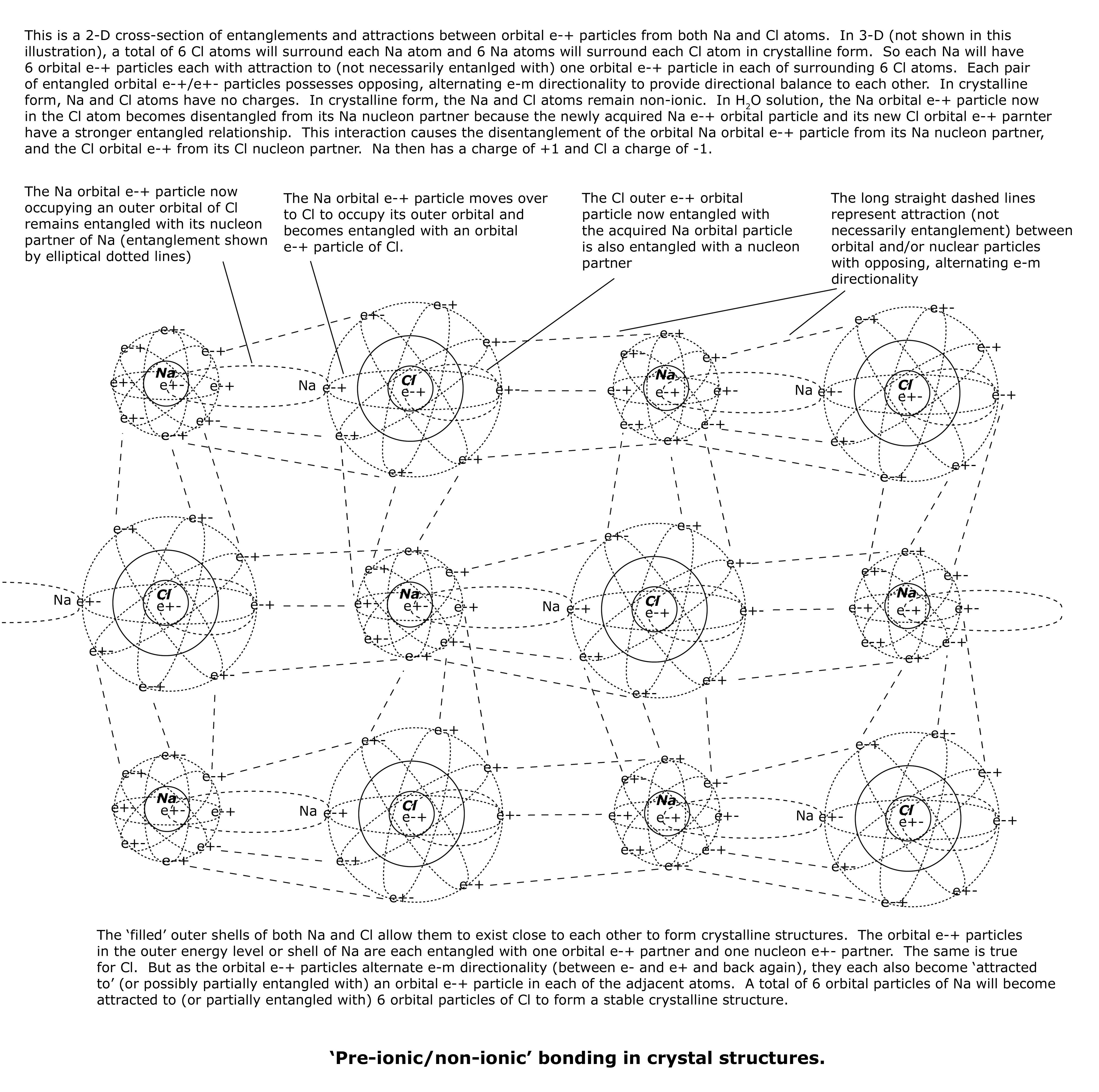
To briefly review, the unpaired outer orbital e-+ particle of Na (metal) has ‘jumped’ over to the outer orbital of Cl (non-metal) to fill its 3p energy level.
The lone outer Na orbital e-+ particle in the 3s energy level that now occupies the outer 3p energy level of Cl stays entangled with its nucleon e+- particle in Na. The nucleon e+- in Na also occupies the outer orbital of the Na nucleus by itself – it has no nucleon partner. So it is only entangled with its orbital e-+ partner that is now part of the Cl atom.
The unpaired Cl outer orbital e-+ particle in 3p is entangled with an unpaired Cl nucleon partner that occupies the outer orbital within the nucleus. This unpaired Cl nucleon is only entangled with its orbital e-+ partner. When the lone outer Na orbital e-+ particle comes over to occupy the 3p energy level of Cl, the unpaired Cl outer orbital e-+ particle also becomes entangled with it.
Now there are two sets of entangled relationships involving the Na and Cl orbital e-+ partners. It is unlikely that the Na and Cl lone nucleons (i.e., not entangled with a nucleon partner) can become entangled since they exist in the nuclei of different atoms. But the following entangled ‘triad’ relationships will most likely exist when Na and Cl are in crystalline form:
- Na 3s orbital e-+ particle is entangled with its Na e+- nucleon occupying a corresponding 3s energy level in the Na nucleus, and is also entangled with the formerly unpaired Cl 3p orbital e-+ particle. This creates an entangled ‘triad’ with two orbital particles and one nucleon.
- Cl 3p orbital e-+ particle is entangled with its Cl e+- nucleon occupying a corresponding 3p energy level in the Cl nucleus, and is also entangled with the newly acquired Na 3s orbital e-+ particle. This too creates an entangled ‘triad’ with two orbital particles and one nucleon.
Each of the sets of three entangled particles, or entangled ‘triads,’ will have one net charge with every e-m interaction. That net charge will alternate between minus (e-) and plus (e+) with every e-m interaction. Since there are two sets of the three entangled particles, if one set has a net charge of minus (e-) and the other has a net charge of plus (e+) with each e-m interaction, then the net charge of the two entangled triads of particles will be ‘neutral’ or ‘directionally balanced.’ This is the case in a crystalline structure of NaCl.
When the ‘electrically neutral’ NaCl forms a crystalline structure, the Na and Cl atoms are in close proximity to each other without ‘outside’ interference from other atoms that might create entangled relationships with Na and Cl atoms or attractive forces that could cause directional imbalance and instability to the NaCl structure.