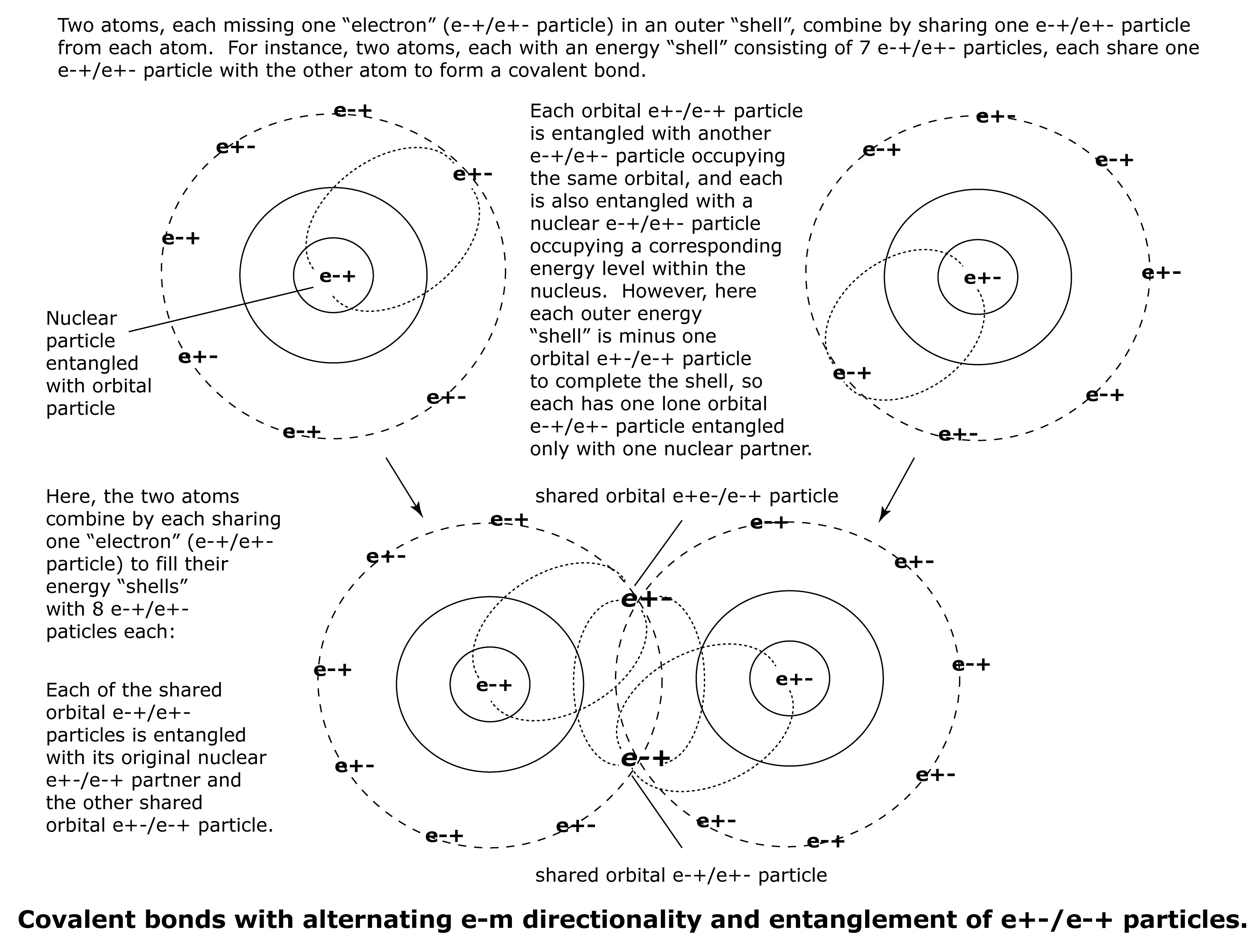
A covalent bond is formed when two atoms, each missing one e-+/e+- particle to fill an energy shell, each share one e-+/e+- particle with the other to fill the energy shells in both atoms.
A covalent bond is formed when two atoms, each missing one e-+/e+- particle to fill an energy shell, each share one e-+/e+- particle with the other to fill the energy shells in both atoms.
Prior to the formation of a covalent bond, each atom has one missing orbital e-+/e+- particle in order to complete or fill an energy shell. This means that each atom has one unpaired orbital e-+/e+- particle, and that particle is entangled only to a nuclear e+-/e-+ partner.
When an e-+/e+- particle is shared in a covalent bond, it remains entangled with its original nuclear e+-/e-+ partner, but also becomes entangled with the shared orbital e+-/e-+ particle from the other atom.
The strength of the covalent bond between the two atoms is due to the entanglement of each shared e-+/e+- particle with a new orbital e+-/e-+ partner of the other atom, while maintaining entanglement with its original nuclear e+-/e-+ partner, thereby being entangled with e+-/e-+ partners from both atoms (one orbital and one nuclear).
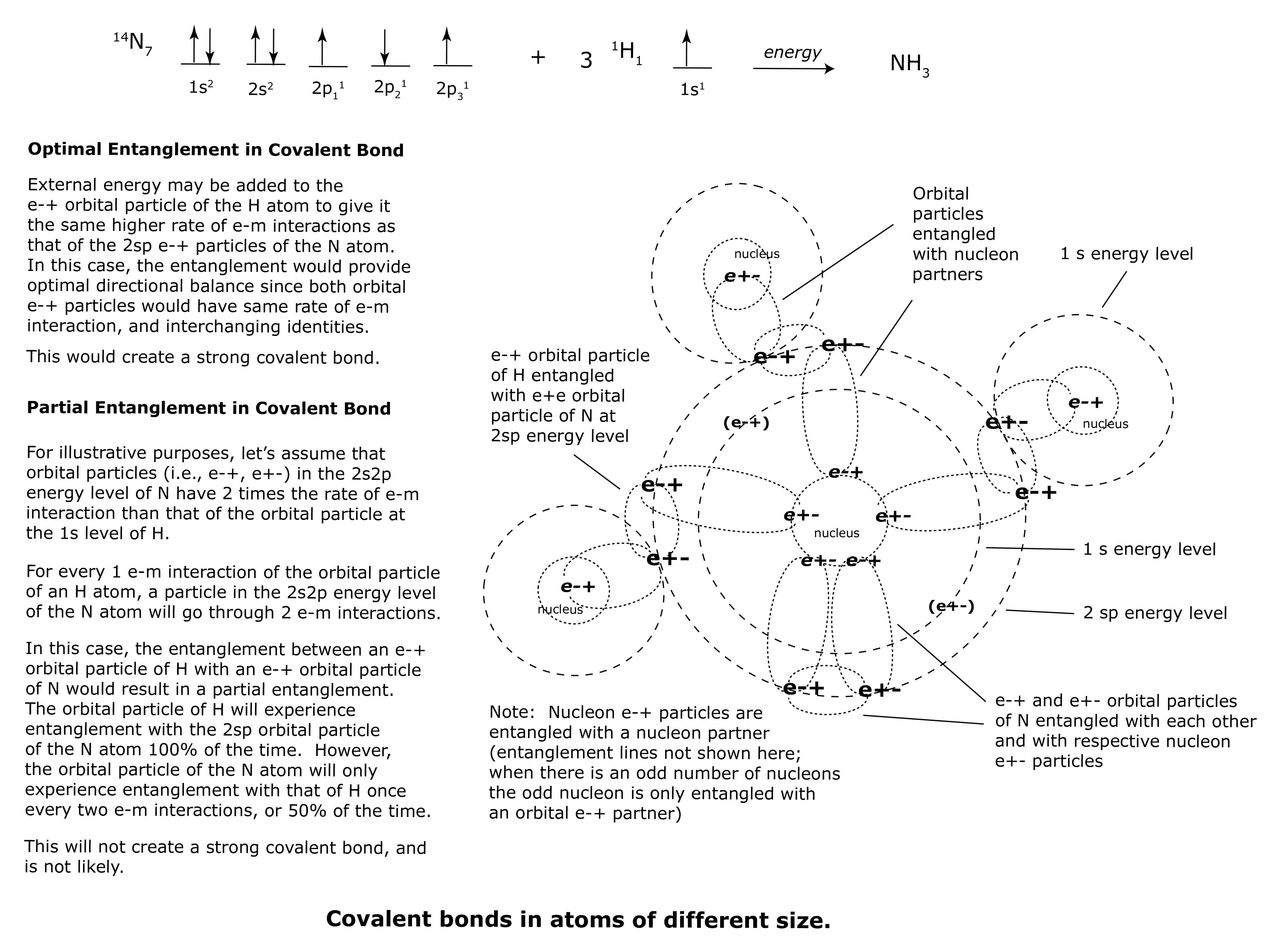
What about covalent bonds with orbital e-+ particles from two different atoms and from two different energy levels? For example, NH3 is composed of one Nitrogen atom and three Hydrogen atoms. The e-+ orbital particles of Hydrogen (H) form covalent bonds with e-+ orbital particles of Nitrogen (N). The e-+ particles of H occupy the 1s energy level while the e-+ particles of N are from the 2sp energy level.
Covalent bonds may be formed when external energy is added to the reaction between Nitrogen and Hydrogen to form NH3. This added external energy may push the e-+ orbital particles of the 1s energy level of H to the same higher rate of e-m interaction as the e-+ orbital particles of the 2sp energy level of N. This would allow the ‘shared’ e-+ and e+- particles of the two elements to be entangled with optimal directional balance. Both the H e-+ orbital particles and the N e-+ orbital particles would possess the same rate of e-m interaction, alternating e-m directionality with every e-m interaction, and interchanging identities. This would result in strong covalent bonds.
On the other hand, if the e-+ orbital particles of the 1s energy level of H possess a lower rate of e-m interactions (let’s say 1/2 the rate, or 50%) than the e-+ orbital particles of the 2sp energy level of N, then they would form a partial entanglement, resulting in weaker covalent bonds.